-
Phase 2/Phase 3
-
-
18+Age Range
-
313Locations
Recruiting
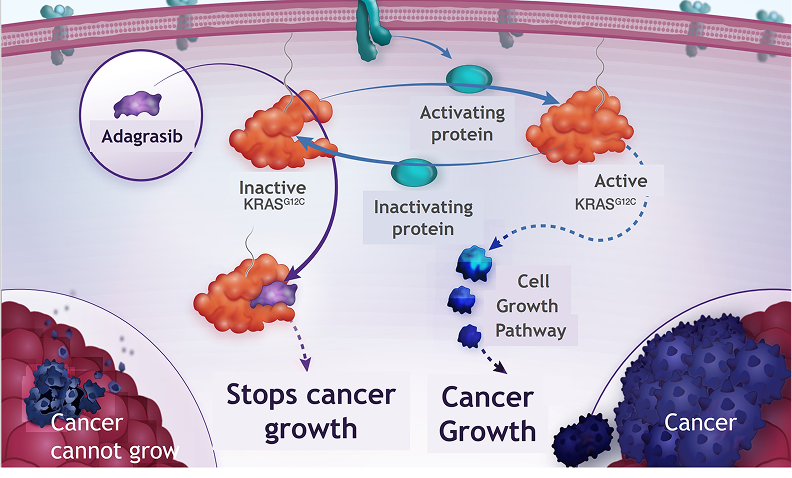
The purpose of this trial is to inform potential treatment options for squamous or non-squamous NSCLC that has spread to other parts of the body (metastatic) or cannot be surgically removed (unresectable). The trial will look at an investigational combination of a drug called adagrasib, which will be used together with immunotherapy (pembrolizumab) in comparison with pembrolizumab alone. This trial is for individuals whose cancer has a genetic change called KRASG12C, whose tumors have a high level of a protein called PD-L1 (50% or higher) and who have not received any treatment for this stage of cancer.
Summary
Nearest Recruiting Site
Download and print the details of this study to bring to your doctor.
Key Eligibility Criteria
Patients who are 18 years or older.
Have been diagnosed with NSCLC after a doctor has looked at a tumor sample under a microscope.
Will be receiving your first treatment for this stage of cancer.
Have metastatic or unresectable NSCLC.
Cancer must have a specific genetic mutation called KRASG12C. The tumors must have a high level of a protein called PD-L1, with a score of 50% or higher. This means that at least 50% of the cancer cells in the tumor exhibit this protein.
Brain metastases untreated up to 2 cm and/or treated and stable.
*There are other requirements for taking part in this clinical trial which the study staff can explain to you. Talk to your doctor or reach out to your nearest study site using the location finder.
Science behind treatment choices
Study Drug Information
What to expect ?
Resources are also available for your healthcare team at KrystalTrialsHCP.com >
 Back to Top
Back to Top