-
Phase 3
-
-
18+Age Range
-
104Locations
Recruiting
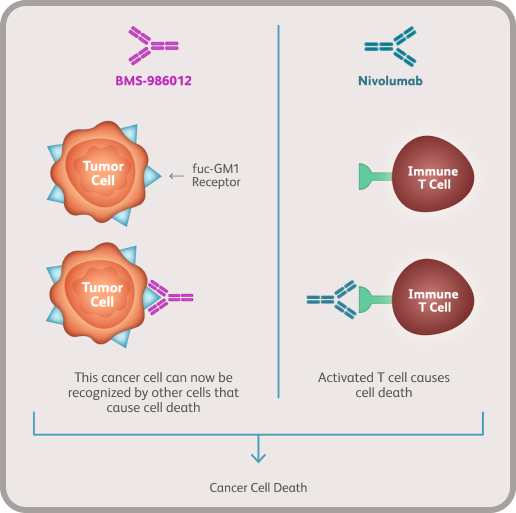
TIGOS is a randomized, double-blind, Phase 3 clinical trial that is testing an investigational drug called BMS-986489. This clinical trial aims to see how safe and effective BMS-986489 is at treating patients with Extensive-stage SCLC. Participants will receive either BMS-986489 in combination with chemotherapy induction followed by BMS-986489 maintenance, or atezolizumab in combination with chemotherapy induction followed by atezolizumab maintenance.
Summary
Nearest Recruiting Site
Download and print the details of this study to bring to your doctor.
Key Eligibility Criteria
Patients who are 18 years or older.
Has confirmed Extensive-stage SCLC.
Can receive platinum-based chemotherapy and a PD-L1-based regimen.
Imaging has shown at least one tumor outside of the central nervous system.
*There are other requirements for taking part in this clinical trial which the study staff can explain to you. Talk to your doctor or reach out to your nearest study site using the location finder.
Study Drug Information
What to expect ?
How will I know if the TIGOS Study is right for me?
Resources are also available for your healthcare team at AtigoStudyHCP.com >
 Back to Top
Back to Top